A better screening for a better care
Our On-the-Spot testing solution

Key advantages
Innovative rapid and portable panel testing

Simple: 50 µl blood tested on an intuitive system

Portable: True On-the-Spot testing

Multiplexed: Up to 8 parameters at once

Safe: Objective results – embedded internal control

Fast: Results in 12 to 18 minutes ( after loading of 1 or 2 cartridges)

Flexible: Capillary or veinous blood, serum, plasma

Generic: One generic platform, adaptable to all immunoassays

Simple: 50 µl blood tested on an intuitive system

Portable: True On-the-Spot testing

Multiplexed: Up to 8 parameters at once

Safe: Objective results – embeded internal control

Fast: Results in 12 to 18 minutes (after loading of 1 or 2 cartridges)

Flexible: Capillary or veinous blood, serum, plasma

Generic: One generic platform / Adaptable to all immunoassays
Our solution
A combination of single use cartridges and a portable instrument for the syndromic Point-of-Care testing
MagIA analyzer
Easy to deploy: Our key for a flexible and simple solution
Our M for screen analyzer presents many benefits:
- Portability: A system weight below 3 kg. Get it done wherever needed
- Autonomy: 2 removable batteries for up to 8 hours off the grid
- Modularity: Analyze either 1 or 2 cartridges in parallel
- Objectivity: unambiguous and qualitative results
M for screen can be upgraded using the following accessories:
- Mini-printer: for patient results printing
- Hand-held scanner: for patient identification



MagIA IBC
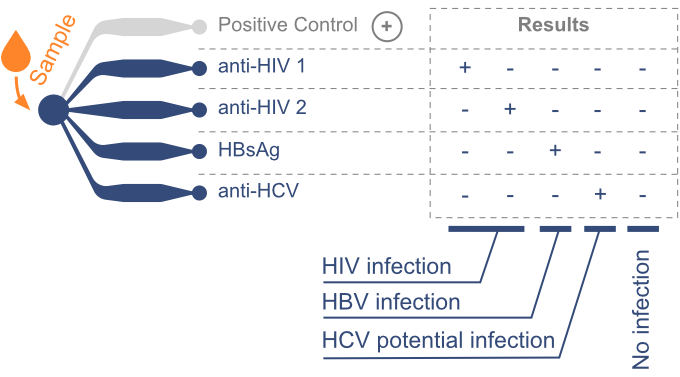
Syndromic screening of HIV, Hepatitis B & C
As per the World Health Organization (WHO), approximately 400 million individuals worldwide are currently infected with HIV and Viral Hepatitis. Each year, there are 4.5 million new infections and 1.8 million fatalities attributed to these diseases. HIV and Viral Hepatitis are pressing global health concerns.
In Europe, there are over 80 million people at risk to be infected. HIV is regularly tested on the spot, whereas Viral Hepatitis testing is usually not included, as most syndromic approaches are limited to laboratory assays and not conducted at the Point-of-Care. Consequently, HIV cases are well-diagnosed, but awareness of viral Hepatitis status remains low, with less than 20% of those infected being aware of their status.
This highlights the need for a combined screening approach at the Point-of-Care to better manage the epidemics and link key populations to care.
In response to this demand, we have developed the MagIA IBC test. This specialized test is designed for the detection of active infections and specifically screens for anti-HIV, anti-HCV, and HBsAg in capillary blood samples.
Therefore, MagIA IBC test enables the simultaneous detection of HIV, Hepatitis B & C infections. Swift and accurate identification of the presence or absence of these pathogens supports timely decision-making regarding treatment and infection control.
Non-contractual visuals / Product not CE marked

Future panels

MagIA’s generic approach allows for the development of countless immunoassays. Our roadmap includes:
STI panel completion:
As a frequent coinfection of HIV and a re-emerging disease, we consider adding syphilis to our STI panel. We also consider completing the panel with p24 for early HIV detection.
Hepatitis B:
By detecting HBsAg as well as anti-HBs and anti-HBc antibodies, the HepB test will enable to make a rapid decision for patient vaccination or treatment.
Tropical fever:
A tropical fever test will allow the parallel detection of Dengue fever, Chikungunya and Zika.
MagIA’s generic approach allows for the development of countless immunoassays. Our roadmap includes:
STI panel completion:
As a frequent coinfection of HIV and a re-emerging disease, we consider adding syphilis to our STI panel. We also consider completing the panel with p24 for early HIV detection.
Hepatitis B:
By detecting HBsAg as well as anti-HBs and anti-HBc antibodies, the HepB test will enable to make a rapid decision for patient vaccination or treatment.
Tropical fever:
A tropical fever test will allow the parallel detection of Dengue fever, Chikungunya and Zika.

Download our
brochure here

Home
Diagnostics
Life sciences
About us
Contact us
News
Legal Notice
Cookies policy
Log in
Home
Diagnostics
Life sciences
About us
Contact us
News
Legal Notice
Cookies policy
Log in
Home
Diagnostics
Life sciences
About us
Contact us
News
Legal Notice
Cookies policy
Log in


